Into the Night
Verified User
It would help greatly if you got help for your mental issues.
Lame. Mantra 1d.
It would help greatly if you got help for your mental issues.
Earth. How about you?
Bulverism!!!!!!!!!!
Never heard of any planet called Bulverism. Where is it?
Bulverism!!!!!!!!!!
This is flat out Bulverism!!!!!!!
So you think Bulverism is flat, instead of round like other planets?
Never heard of any planet called Bulverism. Where is it?
You have no idea why I'm making fun of you and Bulverism do you?
That's good because it is only a guide for general behavior patterns. Raoult's law is an idealized model and will give you results that are reasonably accurate. In practice, however, since what is computed is a temperature change, you necessarily need to know the temperature of the solvent that is diluting the solution as well ... and you'll notice that that's not one of the variables.ΔT(c)=S⋅log (c/ci) Working just as predicted.
ΔT(c)=S⋅log (c/ci)
Working just as predicted.
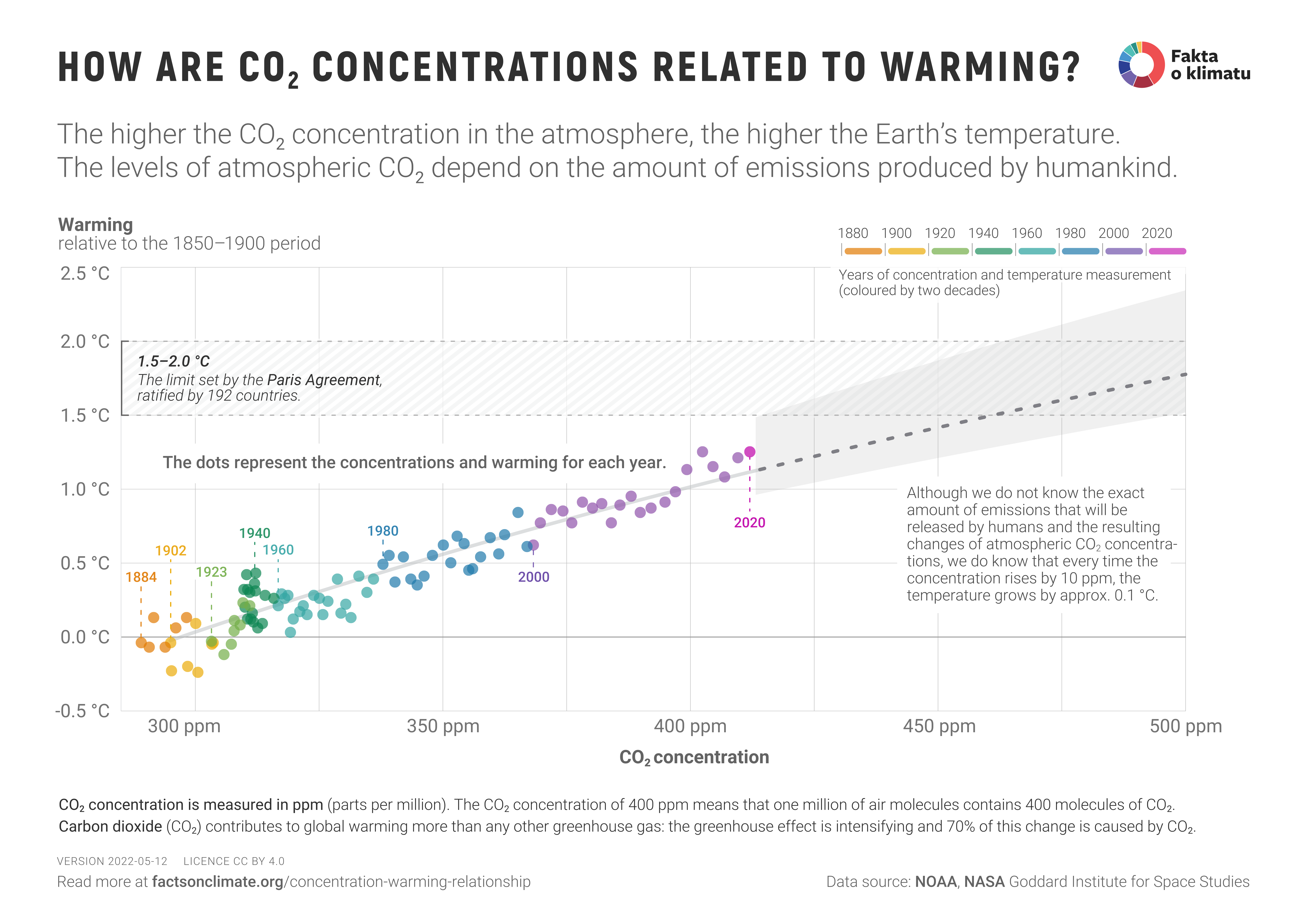
https://factsonclimate.org/assets/generated/concentration-warming-relationship_6000.png
