G
Guns Guns Guns
Guest
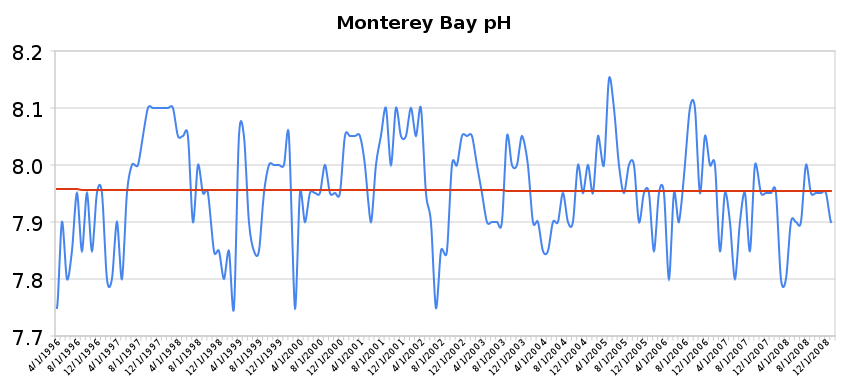
In the last hundred years, rising carbon dioxide from human activities has lowered ocean pH by 0.1 unit, and the Intergovernmental Panel on Climate Change (IPCC) predicts that pH will fall another 0.2 units by 2100, raising the possibility that we may soon see the same sort of ocean changes as those observed during the Paleocene-Eocene Thermal Maximum.
"What we're doing today really stands out in the geologic record," says lead author Bärbel Hönisch, a paleoceanographer at Columbia University's Lamont-Doherty Earth Observatory."
We know that life during past ocean acidification events was not wiped out - new species evolved to replace those that died off. But if industrial carbon emissions continue at the current pace, we may lose organisms we care about - coral reefs, oysters, salmon."
A “bromine explosion” in the Arctic back in 2008 has yielded a disturbing scientific analysis: the replacement of perennial sea-ice with younger seasonal ice could lead to mercury pollution in the Arctic.
In a new NASA-led study, American, Canadian, German and UK researchers believe they have identified the mechanism by which the melting ice cap alters the atmospheric concentration of bromine – and what happens to the bromine afterwards.
The bromine processes – described as bromine explosions by team leader Son Nghiem from the Jet Propulsion Laboratory – take place because of the interaction between sea ice salt and sunlight in the Arctic’s low temperatures.
In the “bromine explosion”, the bromine released by the salty ice creates molecules of bromine monoxide in the atmosphere, which then reacts with gaseous mercury in the atmosphere. The resulting pollutant falls to Earth’s surface.March 2008, by no coincidence, was also the year in which perennial sea-ice reached its lowest recorded level (also discussed in this story). Its loss is partly replaced by seasonal ice, but the younger ice yields more bromine, for two reasons: it hasn’t undergone the leaching process that removes salt from sea ice; and it contains more of the high-salt crystals called “frost flowers”, which provide more salt to fuel bromine releases.
If sea-ice continues to be dominated by younger, saltier ice, Nghiem said, extreme Arctic cold spells will lead to more bromine explosions – and more mercury pollution.
http://www.tgdaily.com/sustainabili...03/02/bromine_explosions_and_surface_mercury/
"What we're doing today really stands out in the geologic record," says lead author Bärbel Hönisch, a paleoceanographer at Columbia University's Lamont-Doherty Earth Observatory."
We know that life during past ocean acidification events was not wiped out - new species evolved to replace those that died off. But if industrial carbon emissions continue at the current pace, we may lose organisms we care about - coral reefs, oysters, salmon."
A “bromine explosion” in the Arctic back in 2008 has yielded a disturbing scientific analysis: the replacement of perennial sea-ice with younger seasonal ice could lead to mercury pollution in the Arctic.
In a new NASA-led study, American, Canadian, German and UK researchers believe they have identified the mechanism by which the melting ice cap alters the atmospheric concentration of bromine – and what happens to the bromine afterwards.
The bromine processes – described as bromine explosions by team leader Son Nghiem from the Jet Propulsion Laboratory – take place because of the interaction between sea ice salt and sunlight in the Arctic’s low temperatures.
In the “bromine explosion”, the bromine released by the salty ice creates molecules of bromine monoxide in the atmosphere, which then reacts with gaseous mercury in the atmosphere. The resulting pollutant falls to Earth’s surface.March 2008, by no coincidence, was also the year in which perennial sea-ice reached its lowest recorded level (also discussed in this story). Its loss is partly replaced by seasonal ice, but the younger ice yields more bromine, for two reasons: it hasn’t undergone the leaching process that removes salt from sea ice; and it contains more of the high-salt crystals called “frost flowers”, which provide more salt to fuel bromine releases.
If sea-ice continues to be dominated by younger, saltier ice, Nghiem said, extreme Arctic cold spells will lead to more bromine explosions – and more mercury pollution.
http://www.tgdaily.com/sustainabili...03/02/bromine_explosions_and_surface_mercury/