Saint Guinefort
Verified User
He certainly can be. I tend to like his dry sense of humor. You, on the other hand, are boring. Your religion is known to both of us. We've heard it all before.

He certainly can be. I tend to like his dry sense of humor. You, on the other hand, are boring. Your religion is known to both of us. We've heard it all before.

I appreciate your post. You and I have something to discuss.Nopers. "Total Organic Carbon (TOC) And It's Measurement"
"Total Organic Carbon Analysis"
Shall I go on?
The first reference makes a solid case to me for having a seemingly contradictory term, i.e.organic carbon.
You are the first person to bring this explanation to my attention. Thank you.
You screwed up with the second reference, however. I imagine that you were searching for titles with "organic carbon" in the title, found one and included it, perhaps without having read it.
The author, Daniel M Jarvie, is a scheister who doesn't understand how hydrocarbons form in the earth and who doesn't understand the Fischer-Tropsch process, but is nonetheless pretending that oil companies and geologists search for oil based on egregious laymen's misconceptions. I stopped reading after a few sentences.
Your first reference was sufficient and I thank you for it.
QM is the basis of Chemistry. It is the heart of chemistry.
It's shocking that someone with a PhD in "biogeochem" would write this.
Chemistry, first and foremost, is the study of the reactions and nature of bonds between atoms and molecules based on their valence shell electron state.
The quantum properties of fundamental particles like leptons, quarks, and the rest of the particle zoo is firmly is within the project of physics.
Bohr, Schrodinger, Heisenberg, Pauli were all physicists.
If you contacted a university and told them you wanted to do a graduate degree in quantum mechanics, 99 times out of 100 they would refer you to the physics department.
It's shocking that someone with a PhD in "biogeochem" would write this.
Chemistry, first and foremost, is the study of the reactions and nature of bonds between atoms and molecules based on their valence shell electron state.
The quantum properties of fundamental particles like leptons, quarks, and the rest of the particle zoo is firmly is within the project of physics.
Bohr, Schrodinger, Heisenberg, Pauli were all physicists.
If you contacted a university and told them you wanted to do a graduate degree in quantum mechanics, 99 times out of 100 they would refer you to the physics department.
Cypress actually thinks physics is wholly separable from chemistry??!?! I’d like to see a link to that if he did indeed claim it. Wow.You don't have any chemistry books anywhere around you? Open one up. You'll find it filled to the brim with QM.
(I am honestly confused why you think physics is somehow wholly separable from chemistry as if that makes a difference. But it is clarifying how astoundingly uneducated you are in this area.)
You don't have any chemistry books anywhere around you? Open one up. You'll find it filled to the brim with QM.
(I am honestly confused why you think physics is somehow wholly separable from chemistry as if that makes a difference. But it is clarifying how astoundingly uneducated you are in this area.)
I love how you just ignore stuff that doesn't comport with your views. LOL.
But then you don't really understand any of this stuff. You're probably as poorly educated as Cypress!
You said quantum mechanics was the "heart" of the science of chemistry.
Chemistry is first and foremost, all about the valence shell electrons, and the reactions and bonds that result from the valence state.
You said quantum mechanics was the "heart" of the science of chemistry.
Nobody who supposedly has a "biogeochem" PhD would write that.
Chemistry is first and foremost, all about the valence shell electrons, and the reactions and bonds that result from the valence state.
Here's a project for you: starting Monday morning, call ten different universities, and assuming you get someone who knows what they're doing, tell them you want to get a graduate degree in quantum mechanics.
All ten universities are going to ultimately refer you to their physics department, not their chemistry or 'biogeochem' department
Just to be clear it isn't some "official chemical definition", it's more of a descriptor. Like I said it is often necessary to know if the carbon you are measuring is from an organic material like a kerogen or a bitumen etc. or if is from CaCO3 (calcium carbonate, eg limestone etc.).
That's the reason for the designation. Otherwise you are 100% correct that all carbon is just plain ol' carbon. This is an "accounting" type of measurement. Where is the carbon you are measuring coming from?
My pleasure.
No, it simply helps establish that the concept of "organic carbon" is well known in the field.
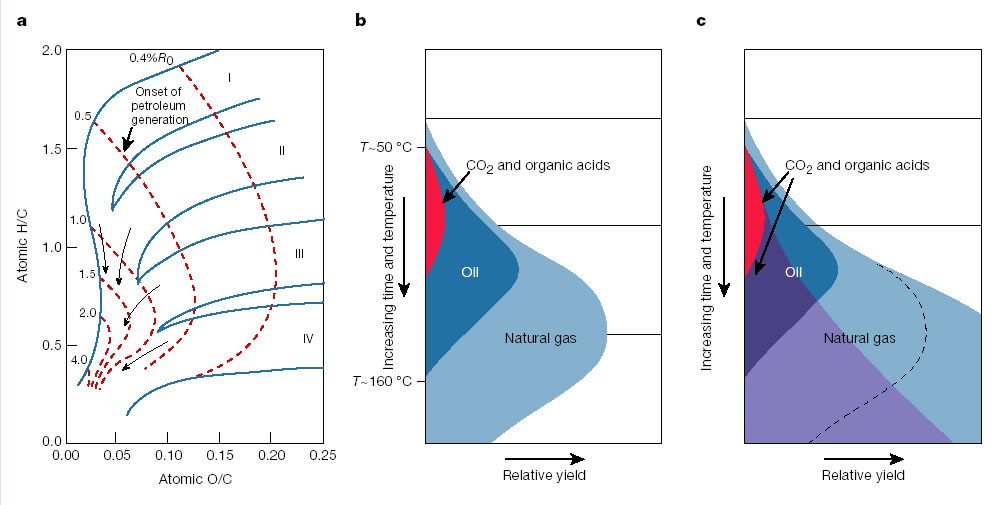
Umm, you were wrong then. The author is, indeed, telling you about where petroleum comes from. It comes from the diagenetic and catagenetic alteration of vegetal/algal/bacterial sources buried deeply and exposed to both aerobic and anaerobic conditions as well as heat. The reference to Tissot and Welte in the first paragraph is a good one. That was a major resource for me when I was learning organic geochem. The Tissot Diagram is actually a great "summary":
(SOURCE)
This basically shows you how various organics are formed in the geologic setting. The X-axis tells you about Oxygen/Carbon ratios and the Y-Axis shows you H/C ratios the "paths" show what happens to these ratios as you increase temperature (the dashed lines are "vitrinite reflectance" values which are a proxy for temperature as measured by the reflectance of coal macerals called "vitrinites"). As you increase temperature you see a steady decline in both O and H as these are volatilized away as the carbon is altered. The three "branches" you see relate to the type of organic material. You can also see in the side-graphs where petroleum forms vs where gas forms etc.
This is kind of the core of organic geochemistry.
No prob.
One of us is definitely wrong, and it's not me.Umm, you were wrong then.
Nope. He is regurgitating a common laymen's misconception. Chemists know how hydrocarbons form. It would seem that many geologists never got the memo and blunder through life thinking that hydrocarbons form from previous organic matter.The author is, indeed, telling you about where petroleum comes from.
Nope. No biological matter is involved. There is nothing preventing you from making any hydrocarbons you want in your garage provided you have the right equipment, some hydrogen, some carbon and the right catalyst (metal). No organic anything will be needed.It comes from the diagenetic and catagenetic alteration of vegetal/algal/bacterial sources buried deeply and exposed to both aerobic and anaerobic conditions as well as heat.
It would appear that "geochemistry" teaches a long-held religious dogma that has long since been debunked.This is kind of the core of organic geochemistry.
So you're not really a 'biogeochem' PhD, are you.What do you think an orbital is???????
You wrote quantum mechanics was the "Heart" of the science of chemistry. Not that there were a couple lectures on it in freshman chemistry.
There is usually never anything more than a passing discussion of wave particle duality and the quantum wave function in introductory chemistry and organic chemistry.
Hey dummy, most of the orbitals aren't involved in chemical reactions, or covalent bonds and ionic bonds.
Chemical reaction and bonding is all about the valence shell electrons and filling up the octet in the valence shell
I leave my standing challenge for you: call ten different universities, tell them you want a graduate degree in quantum mechanics, and observe how all the universities will refer you to their physics department, not chemistry department.
One of us is definitely wrong, and it's not me.
Nope. He is regurgitating a common laymen's misconception. Chemists know how hydrocarbons form. It would seem that many geologists never got the memo and blunder through life thinking that hydrocarbons form from previous organic matter.
This is not the case. If you are operating under this embarrassing misconception, I would make it a priority to ditch it for the correct understanding.
1. Hydrocarbons form much deeper in the crust, in massive quantities, from natural geological activity, from regular carbon and hydrogen, with a metal catalyst, whenever the conditions for the Fischer-Tropsch process exist.
2. Hydrocarbons form in a matter of hours, not millions of years. They form under conditions of elevated temperatures and pressures which ELEVATE the level of chemical energy, which is why they make such awesome fuels. Hydrocarbons are the Earth's best renewable source of energy.
3. Hydrocarbons, once produced by the geological activity described above, rise towards the surface until they encounter impermeable rock and cannot proceed further, thus accumulating into a well. This places hydrocarbon wells BELOW impermeable rock where no organic matter ever reached.
4. The above has long-since been verified by petroleum companies who, after tapping wells dry and capping them, simply wait for them to refill. Then off comes the cap and pumping resumes. Note: millions of years are not needed for wells to refill.
5. Where there are gaps in the impermeable rock, such as in the Gulf of Mexico, the hydrocarbons rise to the surface. Petroleum companies know where to build their offshore platforms by observing where the hydrocarbons are emerging from the sea floor.
Nope. No biological matter is involved. There is nothing preventing you from making any hydrocarbons you want in your garage provided you have the right equipment, some hydrogen, some carbon and the right catalyst (metal). No organic anything will be needed.
It would appear that "geochemistry" teaches a long-held religious dogma that has long since been debunked.
https://m.youtube.com/watch?v=44OU4JxEK4k&t=3s
https://m.youtube.com/watch?v=prtmNHkbtYQ
https://m.youtube.com/watch?v=-I_UtU7zYs0&t=4s
https://m.youtube.com/watch?v=To_RJ_mPNqM&t=57s
You can't say this.Chemistry, first and foremost, is the study of the reactions and nature of bonds between atoms and molecules based on their valence shell electron state.
Sure, but they are highly speculative ... in science terminology we say "purely theoretical." Anyone might be falsifying them tomorrow. Anyone is welcome to use his own conceptual models and notation if it helps.The quantum properties of fundamental particles like leptons, quarks, and the rest of the particle zoo is firmly is within the project of physics.
Not likely. They would tell you that they can offer you a degree in physics or a degree in math, and that you would have to choose which one you wanted, but that you would just need to include a certain concentration in statistical math and a course or two in quantum physics in the physics department.If you contacted a university and told them you wanted to do a graduate degree in quantum mechanics, 99 times out of 100 they would refer you to the physics department.
You can't say this.
Chemistry is the science of matter, period. Tomorrow our existing models of bonds between atoms and molecules might be falsified and replaced with different models. Chemistry will remain the science of matter, whatever the existing models might be.
Looks like I got you frantically googling again.I know you are a lying sack of shit but please, open a chemistry book. Look up "orbital". That's QM. Orbitals are how we understand electrons. Electrons are how elements bond. Bonds are the core of chemistry.
It is all QM. Even bonded they form molecular orbitals which are QM.
I don't know what you think chemistry is but you don't seem to have ever taken a class in it.
THAT IS A LIE. Wow. The lie of all lies. We spend days, weeks, months of lecture on the orbitals. Orbitals are the solutions to the Schroedinger Equation. It don't get more QM than that.
The valence ones sure as fuck are.
And the non-valence electrons also help define the CHEMICAL FEATURES. The size of the atom, the charge/size ratio. This all impacts how elements bond. Remember talking about Si based life? You didn't understand why Si doesn't self-catenate like C does even though both have the same valence configuration. The difference is in the size of the atom. Si has an additional level so the atom is a different size and the bonds it forms differ from C. But both have a 4 electron valence shell.
I am starting to get a clearer picture of your failure in this end of science. You simply didn't have any chemistry classes at all.
Good googling again. Kudos on "octet". But you should also realize there are "expanded valence shells" like in P or S which have more than an octet. But the point is that "octet" is really just the s and p orbitals filling up. 8= 2 s and 6p electrons. It gets more complex when you have d orbitals or f-orbitals which have many more electrons. The whole point being that those s and p and d and f orbitals are quantum mechanics. And when you fill those orbitals remember Hund's rule? Yeah, the SPIN of the electrons has to be paired. That's QM again. 100% QM right down the line. You surely remember how to fill the p orbitals right?
I challenge you to open a chemistry text book. Try learning this stuff.
When leftists shout "education!" they mean "indoctrination of our children into Marxism so we can easily control the next generation!" which of course has no value to anyone except to the leftists shouting "education!" When leftists shout "Support our children!" they mean "Give more power to the teachers' union and alleviate their debilitating accountability to parents for results!"
"Education" is the left's euphemism for "fascism."
Maybe when Into the Night offers to teach you something, you should consider checking your hatred for learning at the door and take some notes. You might find it useful not being as stupid as a 25-lb bag of compost.
