Wrong.
Quantum is discussed at the start and throughout all of chemistry. Even intro. Remember the Quantum Numbers (n, l, ml, ms) and all that talk about spin up and spin down (the little arrows you put in the orbital diagrams? The ones that pointed up and down) those were quantum features.
Here's some examples (If you didn't do a fuck ton of these in intro chemistry then you didn't take intro chemistry any time in the last 60 years)
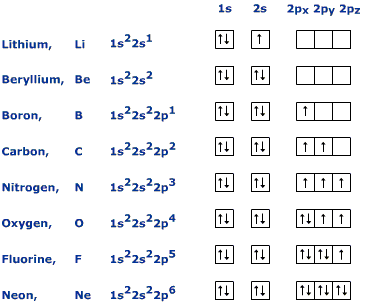
See that "1s, 2s, 2p" stuff?
Those are ORBITALS. The little arrows are electrons. The up pointing ones have different SPIN than the down pointing ones. That's all 100% quantum. Note that you don't just fill them up from left to right. When you get to the p-orbitals you have to fill in all of them with one spin then go back through and fill with the other spin (Hund's rule). That's all quantum.
In the intro chemistry class I had back in '82 we spent a PAINFULLY large amount of time on quantum. Sure we weren't solving the Schroedinger equation or anything but we were dealing with quantum.
Even the Bohr model dealt with quantum concepts (quantization of energy states). But I'm genuinely curious what you think all those orbitals what all those s, p, and d things you had to fill out over and over and over in class.
Remember this little rubric?
What do you think you were doing there? That's quantum mechanics. You are filling ORBITALS in a specific ORDER related to ENERGY of the electron.
How about these?
And what about your minerals classes? Surely you had to learn Pauling's Rules to make it through mineralogy. Those are leveraged off the sizes of atoms. Which, as you know, is related to the electron cloud around the nucleus. Those atoms have the size they do because of...you guessed it...qm. Ever wonder why atoms get SMALLER from left to right across the periodic table? Yup...QM. Filling electrons. Wonder why they get bigger going from top to bottom in the Groups? Yup. Filling electron shells and adding higher shells onto it. QM again.