So? How are they going to change the laws of chemistry and the periodic table?
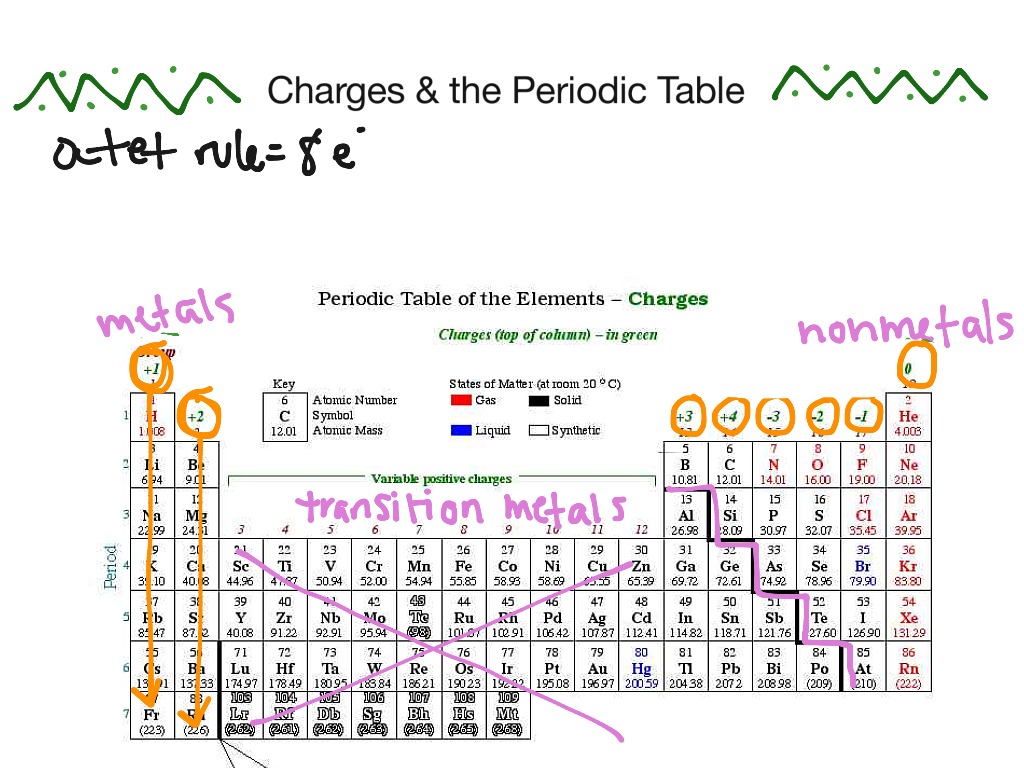
While far from the best, it shows the idea and charges of various elements. Not all are suitable for use in a battery. So, while 6 VDC might theoretically be possible, it's highly unlikely because of the elements involved. Either by their scarcity, reactivity, or other properties you just can't use them. A great example of that is say, Fluorine. Toxic, extremely reactive--makes lithium look safe-- either a gas or liquid at usable temperatures, and just all around dangerous to handle. You aren't using that in a battery.
Heh. I notice that you crossed off lead, which is used in lead-acid batteries. Each cell produces 2.2v.
You also crossed off both nickel and cadmium, used in NiCad batteries. Each cell produces 1.2v.
You also crossed off selenium, which generate a voltage proportional to the amount of light that strikes it.
You also crossed off nickel and iron, used in that NiFe battery you mentioned earlier!
Of the metals, lithium is the lightest (and therefore the most stable). Heavier metals are not used in batteries due to their violent reactive nature, particularly around free oxygen or water.
While you DID allow for carbon, you did cross off zinc, used in common AA cells.
Valence charge is not the resulting voltage available in a battery cell.
The issue with hydrogen is storage.
Yes, but the other and bigger issue with hydrogen is manufacturing it.
A thick steel tank looks like a screen door to hydrogen... Anhydrous ammonia is an alternative. It can be made from natural gas and it's easy to store and relatively safe to handle. If the storage problem can be solved, Hydrogen is the fuel of the future. But we could use ammonia in the meantime.
Ammonia is EXTREMELY dangerous to handle! See the MSDS for anhydrous ammonia!
Three Mile Island was a worst-case scenario for the US nuclear power industry. Nobody died.
There was one death, ruled as an industrial accident (it had nothing to do with the leak itself, other than the chaos in the plant trying to deal with the loss of reactor control). No one died of radiation poisoning or suffered radiation sickness.
Nobody got cancer as a result of the accident.
True.
It was properly contained.
Uh...no. A small amount of radioactive steam was vented to the atmosphere. No one died and no one suffered radiation sickness from it.
At the same time, the operators made egregious mistakes over hours trying to resolve the problems. They did virtually everything wrong.
This DID cause damage to plant equipment and one death (an industrial accident...a worker at the turbine station).
Interestingly, Bessie-Davis in Ohio, the same plant design, had the same original cause of the TMI meltdown--a stuck open relief valve on the pressurizer--occur there about a month earlier than the TMI accident. The Bessie-Davis operators recognized the problem correctly and safely shut the reactor down without further problems.
Quite right.
Chernobyl is irrelevant as it is a graphite moderated, fast fission reactor design used NOWHERE in the Western world because it's only marginally safe and it was being operated in an unsafe and unauthorized manner.
Chernobyl had no containment building either, and they were operating the reactor in an unsafe manner.
Fukushima (I'm leaving out a number of smaller incidents) only saw radioactive material released because it lacked a secondary containment like TMI had.
Fukushima has containment around the reactor, including the destroyed reactor in plant 2. That containment was damaged by the tidal wave that struck the plant. With lack of command and control of the reactor (including the ability to scram it), the reactor melted down and became the twisted mass underwater they are cleaning up today with robots. Fishing was not affected, and no Japanese died from the accident (other than plant workers caught in the tidal wave!). Water itself is a shielding for nuclear reactions, and the ocean is no exception.
That is, at TMI the reactor was inside a large, concrete containment building that was damn near bomb proof. At Fukushima, the reactors were in what amounts to big tin sheds.
The tin sheds housed the concrete containment.
Even at Fukushima, the dangers of the accident were limited almost entirely to the site and as with TMI evacuations were mostly done out of an abundance of caution.
It was and is still limited entirely to the site.
The tidal wave did far more damage and killed a lot more people than Fukushima did!
Oh, did you know that cleaning up TMI cost way, way, less than the BP Deepwater Horizon oil platform disaster in the Caribbean?
True, mostly because TMI is easier to get to.
As for uranium mining, it's mostly done in underground mines and uranium ore is only a health hazard if you stand next to it for something like months on end.
Mining is always hazardous, regardless of the material being mined.
And a light one at that. Regular clothing stops it.
Lithium mining (for batteries) is far, far more nasty simply by the nature of the process.
True. Lithium mining uses open pit mining or sand extraction mining, both of which are messy. Sand extraction requires literally years of processing to get usable ore.
Uranium mine in Arizona
Lithium recovery after ore is mined in US
The mining itself is usually open pit with massive tailings.
This is not an open pit mine. It's a sand extraction mine. These ponds will be leaching lithium for years to produce usable ore. They contain sulfuric acid.
Natural gas is only necessary with nuclear power to run as peaking plants for intermittent use during heavy load periods.
Natural gas is plentiful and much cheaper than nuclear power. It's also a renewable fuel.
So, the relatively small amount of CO2 released is pretty much irrelevant compared to anything currently in use.
It is completely irrelevant as far as Earth's temperature is concerned.